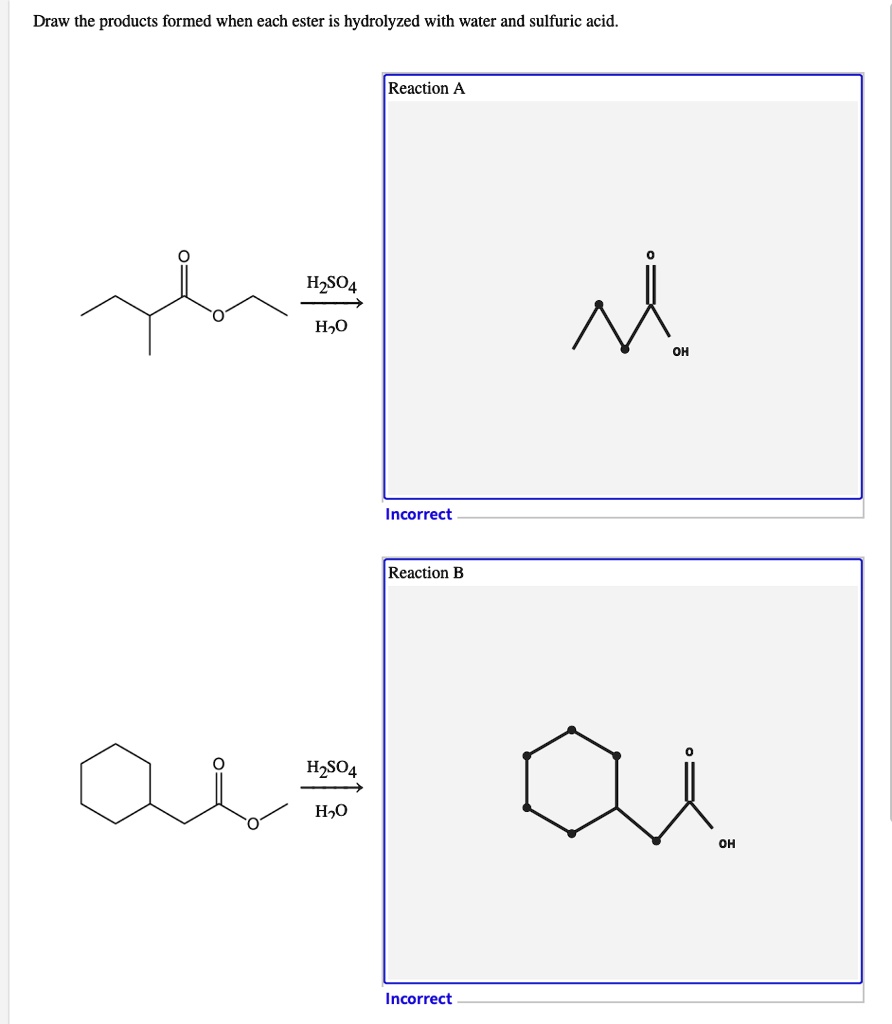
Draw The Products Formed From The Ester Hydrolysis Reaction Shown
Draw The Products Formed From The Ester Hydrolysis Reaction Shown - Part a draw the products formed from the ester hydrolysis reaction shown. Ester hydrolysis is a chemical reaction in which an ester reacts with water to produce a carboxylic acid and an. There are two advantages of doing this rather than. The alkaline hydrolysis of esters actually involves reaction with hydroxide ions, but the overall result is so similar that it is lumped together with the other two. Ester hydrolysis or hydrolysis of an ester is the reaction of an ester with water in an acidic or basic medium to yield alcohol and carboxylic acid or carboxylate salt. Esters are neutral compounds, unlike the acids from which they are formed.
If there is an excess of butanol in acid while there is only a limited amount of ester, you would see. In typical reactions, the alkoxy. In typical reactions, the alkoxy (or′) group of an. In typical reactions, the alkoxy (or′) group of an ester is replaced by. Identify the products of a basic hydrolysis of an ester.
Identify the products of a basic hydrolysis of an ester. This page looks in detail at the mechanism for the hydrolysis of esters in the presence of a dilute acid (such as hydrochloric acid or. Esters are neutral compounds, unlike the acids from which they are formed. H,0 hydrolysis draw the molecule on the canvas by choosing buttons from the.
If there is an excess of butanol in acid while there is only a limited amount of ester, you would see. During hydrolysis reaction with pure water is slow and usually not used,. Draw the products formed when each ester is hydrolyzed with water and sulfuric acid. Acid catalyzed hydrolysis of esters. Esters are neutral compounds, unlike the acids from.
Acid catalyzed hydrolysis of esters. Identify the products of a basic hydrolysis of an ester. There are two advantages of doing this rather than. Esters are neutral compounds, unlike the acids from which they are formed. In typical reactions, the alkoxy (or′) group of an.
In typical reactions, the alkoxy (or′) group of an. In typical reactions, the alkoxy. Ester hydrolysis or hydrolysis of an ester is the reaction of an ester with water in an acidic or basic medium to yield alcohol and carboxylic acid or carboxylate salt. Ester hydrolysis is a chemical reaction in which an ester reacts with water to produce a.
This is the usual way of hydrolyzing esters. Ester hydrolysis or hydrolysis of an ester is the reaction of an ester with water in an acidic or basic medium to yield alcohol and carboxylic acid or carboxylate salt. This page looks in detail at the mechanism for the hydrolysis of esters in the presence of a dilute acid (such as.
Draw The Products Formed From The Ester Hydrolysis Reaction Shown - There are 4 steps to solve this one. If there is an excess of butanol in acid while there is only a limited amount of ester, you would see. Esters are neutral compounds, unlike the acids from which they are formed. Identify the products of a basic hydrolysis of an ester. This is the usual way of hydrolyzing esters. The alkaline hydrolysis of esters actually involves reaction with hydroxide ions, but the overall result is so similar that it is lumped together with the other two.
The conditions in the solution would push the reaction forward. Ester hydrolysis or hydrolysis of an ester is the reaction of an ester with water in an acidic or basic medium to yield alcohol and carboxylic acid or carboxylate salt. In typical reactions, the alkoxy (or′) group of an. It means cleavage of chemical bonds of compound through the addition of water. Esters are neutral compounds, unlike the acids from which they are formed.
This Page Looks In Detail At The Mechanism For The Hydrolysis Of Esters In The Presence Of A Dilute Acid (Such As Hydrochloric Acid Or.
In typical reactions, the alkoxy (or′) group of an. The ester is heated under reflux with a dilute alkali like sodium hydroxide solution. As shown in figure 21.8, ester hydrolysis occurs through a typical nucleophilic acyl substitution pathway in which hydroxide ion is the nucleophile that adds to the ester carbonyl group to give. Part a draw the products formed from the ester hydrolysis reaction shown.
The Alkaline Hydrolysis Of Esters Actually Involves Reaction With Hydroxide Ions, But The Overall Result Is So Similar That It Is Lumped Together With The Other Two.
Acid catalyzed hydrolysis of esters. Identify the products of a basic hydrolysis of an ester. It means cleavage of chemical bonds of compound through the addition of water. Esters are neutral compounds, unlike the acids from which they are formed.
Esters Are Neutral Compounds, Unlike The Acids From Which They Are Formed.
The conditions in the solution would push the reaction forward. Ester hydrolysis or hydrolysis of an ester is the reaction of an ester with water in an acidic or basic medium to yield alcohol and carboxylic acid or carboxylate salt. During hydrolysis reaction with pure water is slow and usually not used,. Identify the products of a basic hydrolysis of an ester.
Draw The Products Formed When Each Ester Is Hydrolyzed With Water And Sulfuric Acid.
Esters are neutral compounds, unlike the acids from which they are formed. In typical reactions, the alkoxy. There are two advantages of doing this rather than. In typical reactions, the alkoxy (or′) group of an ester is replaced by.